What Do Homeobox Genes Code For In Animals?
Zebrafish Development
From Embryology
Leap to:navigation, search
| Embryology - 22 Jun 2022 |
|---|
| Google Translate - select your language from the list shown below (this volition open a new external page) |
| العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may not be accurate. (More? About Translations) |
Introduction
![]()
Zebrafish or zebra danio (danio rerio) are seen as i of the latest "models" for vertebrate embryological development studies. These embryos accept the great advantage that they develop every bit "see through" embryos, that is, all internal development can be conspicuously observed from the outside in the living embryo. Much of the early modern work using this embryo model began with the papers of Kimmel.[1] [two]
Several big laboratories in the The states are now developing large breeding programs to carry out "knockouts" and to detect spontaneous mutants of interest.
Some Recent Findings
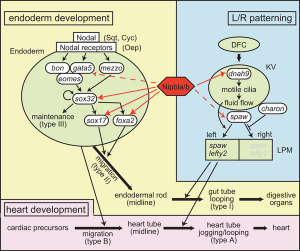
Nipbl eye and organ patterning[3]
- ZebraShare: a new venue for rapid broadcasting of zebrafish mutant data [4] "Background: In the past decade, the zebrafish community has widely embraced targeted mutagenesis technologies, resulting in an abundance of mutant lines. While many lines take proven to be useful for investigating gene function, many have too shown no apparent phenotype, or phenotypes not of interest to the originating lab. In order for labs to document and share information about these lines, we have created ZebraShare as a new resource offered within ZFIN. ZebraShare involves a form-based submission process generated by ZFIN. The ZebraShare interface (https://zfin.org/action/zebrashare) can exist accessed on ZFIN under "Submit Information".
- Involvement of homeobox transcription factor Mohawk in palatogenesis [5] "Palatogenesis is afflicted past many factors, including factor polymorphisms and exposure to toxic chemicals during sensitive developmental periods. Cleft palate is one of the nearly mutual congenital anomalies, and ongoing efforts to elucidate the molecular mechanisms underlying palatogenesis are providing useful insights to reduce the take a chance of this disorder. To identify novel potential regulators of palatogenesis, we analyzed public transcriptome datasets from a mouse model of crevice palate acquired by selective deletion of transforming growth cistron-β (TGFβ) receptor type two in cranial neural crest cells. We identified the homeobox transcription factor Mohawk (Mkx) equally a gene downregulated in the maxilla of TGFβ knockout mice compared with wild-type mice. To examine the role of mkx in palatogenesis, we used CRISPR/Cas9 editing to generate zebrafish with impaired expression of mkxa and mkxb, the zebrafish homologs of Mkx. We institute that mkx crispants expressed reduced levels of gli1, a disquisitional transcription gene in the Sonic hedgehog (SHH) signaling pathway that plays an important role in the regulation of palatogenesis."
- Conserved Genoarchitecture of the Basal Hypothalamus in Zebrafish Embryos [6] "Analyses of genoarchitecture recently stimulated substantial revisions of anatomical models for the developing hypothalamus in mammalian and other vertebrate systems. The prosomeric model proposes the hypothalamus to exist derived from the secondary prosencephalon, and to consist of alar and basal regions. The basal hypothalamus tin further be subdivided into tuberal and mamillary regions, each with singled-out subregions. Albeit being a widely used model arrangement for neurodevelopmental studies, no detailed genoarchitectural maps exist for the zebrafish (Danio rerio) hypothalamus. Here, we compare expression domains of zebrafish genes, including arxa, Shha, otpa, isl1, lhx5, nkx2.1, nkx2.2a, pax6, and dlx5a, the orthologs of which delimit specific subregions within the murine basal hypothalamus. We develop the highly conserved brain-specific homeobox (bsx) gene equally a novel marker for genoarchitectural analysis of hypothalamic regions. Our comparison of factor expression patterns reveals that the genoarchitecture of the basal hypothalamus in zebrafish embryos 48 hours post fertilization is highly similar to mouse embryos at E13.5. We constitute the tuberal hypothalamus in zebrafish embryos to be relatively large and to incorporate previously sick-defined regions effectually the posterior hypothalamic recess. The mamillary hypothalamus is smaller and concentrates to rather medial areas in proximity to the anterior end of the neural tube floor plate. Inside the basal hypothalamus we identified longitudinal and transverse tuberal and mamillary subregions topologically equivalent to those previously described in other vertebrates. Yet, the hypothalamic diencephalic purlieus region and the posterior tuberculum withal provide a challenge. Nosotros practical the updated prosomeric model to the developing zebrafish hypothalamus to facilitate cantankerous-species comparisons. Accordingly, we applied the mammalian nomenclature of hypothalamic organization to zebrafish and propose information technology to supervene upon some controversial previous nomenclature."
- Review - Zebrafish as a model for studying ovarian development: Contempo advances from targeted gene knockout studies [vii] "Ovarian development is a complex process controlled by precise coordination of multiple factors. The targeted cistron knockout technique is a powerful tool to study the functions of these factors. The successful awarding of this technique in mice in the past 3 decades has significantly enhanced our agreement on the molecular mechanism of ovarian development. Recently, with the advent of genome editing techniques, targeted gene knockout enquiry can be carried out in many species. Zebrafish has emerged equally an excellent model arrangement to study the control of ovarian development. Dozens of genes related to ovarian development have been knocked out in zebrafish in recent years. Much new data and perspectives on the molecular mechanism of ovarian development take been obtained from these mutant zebrafish. Some findings have challenged conventional views. Several genes have been identified for the beginning time in vertebrates to command ovarian development. Focusing on ovarian development, the purpose of this review is to briefly summarize recent findings using these cistron knockout zebrafish models, and compare these findings with mammalian models." ovary
- Anatomy, development, and plasticity of the neurosecretory hypothalamus in zebrafish [8] "The paraventricular nucleus (PVN) of the hypothalamus harbors diverse neurosecretory cells with disquisitional physiological roles for the homeostasis. Decades of inquiry in rodents have provided a large amount of data on the anatomy, development, and part of this important hypothalamic nucleus. However, since the hypothalamus lies deep within the brain in mammals and is difficult to access, many questions regarding development and plasticity of this nucleus yet remain. In particular, how different environmental conditions, including stress exposure, shape the evolution of this important nucleus has been difficult to address in animals that develop in utero. To accost these open questions, the transparent larval zebrafish with its rapid external development and splendid genetic toolbox offers exciting opportunities. In this review, we summarize recent information on the anatomy and development of the neurosecretory preoptic surface area (NPO), which represents a similar structure to the mammalian PVN in zebrafish. We will then review recent studies on the development of unlike cell types in the neurosecretory hypothalamus both in mouse and in fish. Lastly, we discuss stress-induced plasticity of the PVN mainly discussing the data obtained in rodents, but pointing out tools and approaches available in zebrafish for future studies. This review serves as a primer for the currently available information relevant for studying the development and plasticity of this important brain region using zebrafish." hypothalamus
| More than contempo papers |
|---|
| This tabular array allows an automatic computer search of the external PubMed database using the listed "Search term" text link.
More? References | Discussion Page | Journal Searches | 2019 References | 2020 References Search term: Zebrafish Embryology | Zebrafish Evolution |
| Older papers |
|---|
| These papers originally appeared in the Some Recent Findings tabular array, simply as that list grew in length have now been shuffled down to this collapsible table. See also the Discussion Page for other references listed by year and References on this current page.
|
Movies
| Picture show of an immobilized zebrafish embryo development from the 1-cell stage to 85 hours postal service fertilisation (hpf).[17] |
Zebrafish Stages
| Elapsing | Flow Proper noun | Image | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 - 0.75 hrs | Zygote Catamenia |  | |||||||||
| 0.75 - ii.25 hrs | Cleavage Period |  | |||||||||
| two.25 - 5.25 hrs | Blastula Menstruum | 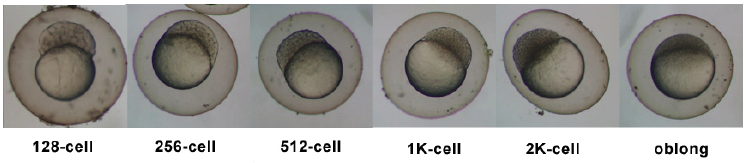 | |||||||||
| 5.25 - 10.33 hrs | Gastrula Catamenia | 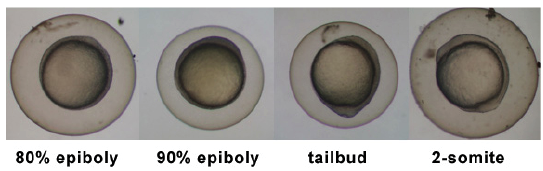 | |||||||||
| 10.33 - 24 hrs | Partition Menstruation | 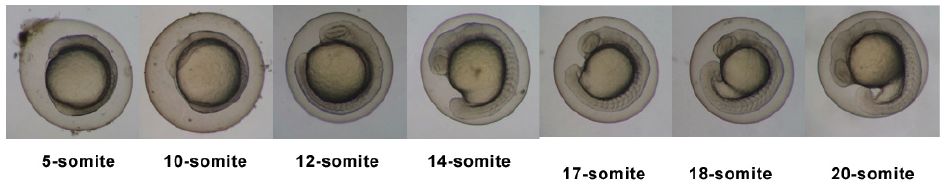 | |||||||||
| 24 - 48 hrs | Pharyngula Period | 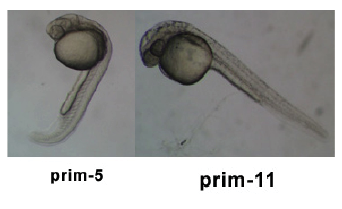 | |||||||||
| 48-72 hrs | Hatching Menstruation |  | |||||||||
| 72 hrs - 30 Days | Larval Period | 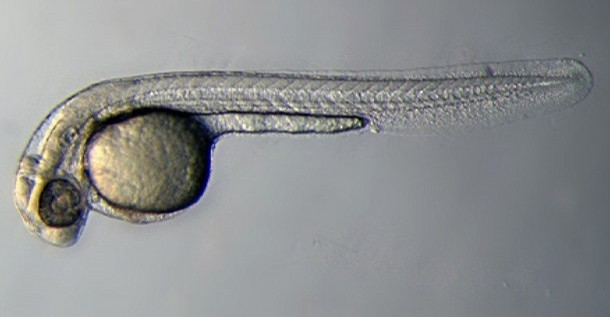 | |||||||||
Links: zebrafish | zebrafish timeline
Image Source: Epitome kindly contributed by Dr Judith Cebra-Thomas Department of Biology, Millersville University. From her Lab Protocols showing Zebrafish Stages sequence based on previously published sources including; Kimmel CB, Ballard WW, Kimmel SR, Ullmann B & Schilling TF. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn. , 203, 253-310. PMID: 8589427 DOI. | |||||||||||
Pharyngula Period
- Transition from Prim 5 to Long-pec
- The torso axis begins to straighten and the caput straightens out and lifts dorsally
- Notochord is well adult
- Formation of the Dorsal and Ventral Stripe
- Nervous arrangement is hollow and expanding anteriorly
- The encephalon has developed into 5 singled-out lobes
- Seven pharyngeal curvation's develop rapidly during this stage
- Pectoral fins begin to develop
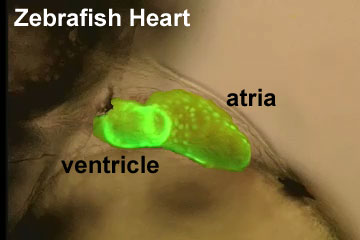
- The Circulatory system develops and the heart beats for the first time
- Blood begins to circulate through a closed circuit of channels
- Tactile sensitivity appears and uncoordinated movements occur
-
day one
-
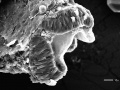
brain fold
-

myotomes
-

trunk
-
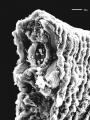
trunk
-
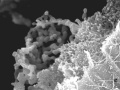
perichordal sheath
-

enveloping layer
-

enveloping layer
Skull
 | Zebrafish Skull Neural Crest Contribution [18]
|
Neural
Sensory
Lateral line is a zebrafish sensory system, used to discover changes in water flow, composed of clusters of mechanosensory hair cells called neuromasts.
Molecular
Fibroblast Growth Cistron
- Fgf8 and Fgf3 - regulating the segmentation of the pharyngeal endoderm into pouches.[19]
- Fgf24 and Fgf8 - promotes posterior mesodermal development.[20]
- Sox9 - required for cartilage morphogenesis.[21]
References
- ↑ Kimmel CB, Sessions SK & Kimmel RJ. (1981). Morphogenesis and synaptogenesis of the zebrafish Mauthner neuron. J. Comp. Neurol. , 198, 101-20. PMID: 7229136 DOI.
- ↑ Kimmel CB, Sepich DS & Trevarrow B. (1988). Development of segmentation in zebrafish. Development , 104 Suppl, 197-207. PMID: 3077108
- ↑ three.0 3.1 Muto A, Calof AL, Lander Advertising & Schilling TF. (2011). Multifactorial origins of heart and gut defects in nipbl-scarce zebrafish, a model of Cornelia de Lange Syndrome. PLoS Biol. , 9, e1001181. PMID: 22039349 DOI.
- ↑ DeLaurier A, Howe DG, Ruzicka L, Carte du jour AN, Mishoe Hernandez L, Wiggins KJ, Gallati MM, Vanpelt Thou, Loyo Rosado F, Pugh KG, Shabdue CJ, Jihad Grand, Thyme SB & Talbot JC. (2021). ZebraShare: a new venue for rapid dissemination of zebrafish mutant data. PeerJ , nine, e11007. PMID: 33954026 DOI.
- ↑ Adachi Y, Higuchi A, Wakai Eastward, Shiromizu T, Koiwa J & Nishimura Y. (2021). Interest of homeobox transcription factor Mohawk in palatogenesis. Congenit Anom (Kyoto) , , . PMID: 34816492 DOI.
- ↑ Schredelseker T & Driever W. (2020). Conserved Genoarchitecture of the Basal Hypothalamus in Zebrafish Embryos. Front Neuroanat , xiv, iii. PMID: 32116574 DOI.
- ↑ Li J & Ge West. (2020). Zebrafish as a model for studying ovarian evolution: Recent advances from targeted gene knockout studies. Mol. Prison cell. Endocrinol. , 507, 110778. PMID: 32142861 DOI.
- ↑ Nagpal J, Herget U, Choi MK & Ryu S. (2019). Anatomy, development, and plasticity of the neurosecretory hypothalamus in zebrafish. Cell Tissue Res. , 375, 5-22. PMID: 30109407 DOI.
- ↑ Pennisi E. (2018). Development jail cell by prison cell. Science , 362, 1344-1345. PMID: 30573610 DOI.
- ↑ Li J, Gao F, Zhao Y, He L, Huang Y, Yang X, Zhou Y, Yu L, Zhao Q & Dong X. (2019). Zebrafish znfl1s regulate left-right disproportion patterning through controlling the expression of fgfr1a. J. Prison cell. Physiol. , 234, 1987-1995. PMID: 30317609 DOI.
- ↑ Naylor RW, Qubisi SS & Davidson AJ. (2017). Zebrafish Pronephros Development. Results Probl Cell Differ , sixty, 27-53. PMID: 28409341 DOI.
- ↑ Antinucci P & Hindges R. (2016). A crystal-clear zebrafish for in vivo imaging. Sci Rep , half dozen, 29490. PMID: 27381182 DOI.
- ↑ Xu PF, Houssin Northward, Ferri-Lagneau KF, Thisse B & Thisse C. (2014). Construction of a vertebrate embryo from 2 opposing morphogen gradients. Science , 344, 87-9. PMID: 24700857 DOI.
- ↑ Eames BF, DeLaurier A, Ullmann B, Huycke TR, Nichols JT, Dowd J, McFadden M, Sasaki MM & Kimmel CB. (2013). FishFace: interactive atlas of zebrafish craniofacial evolution at cellular resolution. BMC Dev. Biol. , xiii, 23. PMID: 23714426 DOI.
- ↑ Vesterlund L, Jiao H, Unneberg P, Hovatta O & Kere J. (2011). The zebrafish transcriptome during early evolution. BMC Dev. Biol. , xi, 30. PMID: 21609443 DOI.
- ↑ Carney TJ, Feitosa NM, Sonntag C, Slanchev K, Kluger J, Kiyozumi D, Gebauer JM, Coffin Talbot J, Kimmel CB, Sekiguchi K, Wagener R, Schwarz H, Ingham PW & Hammerschmidt Chiliad. (2010). Genetic analysis of fin development in zebrafish identifies furin and hemicentin1 equally potential novel fraser syndrome disease genes. PLoS Genet. , half-dozen, e1000907. PMID: 20419147 DOI.
- ↑ Swinburne IA, Mosaliganti KR, Dark-green AA & Megason SG. (2015). Improved Long-Term Imaging of Embryos with Genetically Encoded α-Bungarotoxin. PLoS ONE , 10, e0134005. PMID: 26244658 DOI.
- ↑ Kague E, Gallagher M, Shush Southward, Parsons M, Franz-Odendaal T & Fisher S. (2012). Skeletogenic fate of zebrafish cranial and torso neural crest. PLoS One , 7, e47394. PMID: 23155370 DOI.
- ↑ Crump JG, Maves L, Lawson ND, Weinstein BM & Kimmel CB. (2004). An essential role for Fgfs in endodermal pouch germination influences subsequently craniofacial skeletal patterning. Development , 131, 5703-16. PMID: 15509770 DOI.
- ↑ Draper BW, Stock DW & Kimmel CB. (2003). Zebrafish fgf24 functions with fgf8 to promote posterior mesodermal development. Development , 130, 4639-54. PMID: 12925590 DOI.
- ↑ Yan YL, Miller CT, Nissen RM, Vocalist A, Liu D, Kirn A, Draper B, Willoughby J, Morcos PA, Amsterdam A, Chung BC, Westerfield M, Haffter P, Hopkins N, Kimmel C, Postlethwait JH & Nissen R. (2002). A zebrafish sox9 gene required for cartilage morphogenesis. Evolution , 129, 5065-79. PMID: 12397114
Journals
Zebrafish "is the only peer-reviewed periodical to focus on the zebrafish, which has numerous valuable features every bit a model organism for the report of vertebrate development. Due to its prolific reproduction and the external development of the transparent embryo, the zebrafish is a prime model for genetic and developmental studies, equally well every bit research in toxicology and genomics. While genetically more distant from humans, the vertebrate zebrafish nevertheless has comparable organs and tissues, such as heart, kidney, pancreas, bones, and cartilage." [jour PubMed listing]
Reviews
Supatto W & Vermot J. (2011). From cilia hydrodynamics to zebrafish embryonic development. Curr. Top. Dev. Biol. , 95, 33-66. PMID: 21501748 DOI.
Carvalho L & Heisenberg CP. (2010). The yolk syncytial layer in early zebrafish development. Trends Cell Biol. , 20, 586-92. PMID: 20674361 DOI.
Brittijn SA, Duivesteijn SJ, Belmamoune M, Bertens LF, Biting W, de Bruijn JD, Champagne DL, Cuppen Due east, Flik G, Vandenbroucke-Grauls CM, Janssen RA, de Jong IM, de Kloet ER, Kros A, Meijer AH, Metz JR, van der Sar AM, Schaaf MJ, Schulte-Merker Due south, Spaink HP, Tak PP, Verbeek FJ, Vervoordeldonk MJ, Vonk FJ, Witte F, Yuan H & Richardson MK. (2009). Zebrafish development and regeneration: new tools for biomedical research. Int. J. Dev. Biol. , 53, 835-fifty. PMID: 19557689 DOI.
Bakkers J, Verhoeven MC & Abdelilah-Seyfried S. (2009). Shaping the zebrafish heart: from left-right centrality specification to epithelial tissue morphogenesis. Dev. Biol. , 330, 213-twenty. PMID: 19371733 DOI.
Chan TM, Longabaugh W, Bolouri H, Chen HL, Tseng WF, Chao CH, Jang TH, Lin YI, Hung SC, Wang HD & Yuh CH. (2009). Developmental gene regulatory networks in the zebrafish embryo. Biochim. Biophys. Acta , 1789, 279-98. PMID: 18992377 DOI.
Articles
Warga RM & Kane DA. (2018). A Wilson cell origin for Kupffer's vesicle in the zebrafish. Dev. Dyn. , , . PMID: 30016568 DOI.
Search Pubmed
Search Pubmed: Zebrafish Development
Additional Images
-

Zebrafish 24-hour interval 1 SEM
-
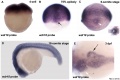
Wdr18 expression
-
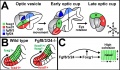
Wdr18 expression six somite phase
-

Zebrafish brain fold
-
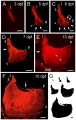
Stages primordial germ jail cell migration
-
Retinal patterning model
-

Non-mammalian VEGF Receptors
-
Bone growth
Terms
- deep cell layer - (DEL) formed afterward blastula stage that forms the 3 germ layers (ectoderm, mesoderm, and endoderm).
- epiboly - (Greek, "epibol" = a throwing or laying on) Term describing the division and movement of ectodermal cells during gastrulation, thinning and spreading this layer to cover the whole of the embryo. Cellular movements are thought to occur in all vertebrates, just have been nearly clearly identified in both the zebrafish and frog (xenopus laevis).
- enveloping layer - (EVL) an epithelial monolayer formed after blastula phase that undergoes epiboly.
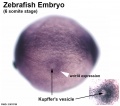
- Kupffer'southward vesicle - (ciliated organ of asymmetry, primitive node) a transient epithelial fluid-filled sac located midventrally posterior to the yolk cell or its extension. The vesicle has been described as equivalent to the archaic node for establishing embryo left-correct (L-R) centrality. PMID 21876750 PMID 30016568
- yolk syncytial layer - (YSL) membrane-enclosed group of nuclei that lie on top of the yolk cell formed after blastula stage that undergoes epiboly.
External Links
External Links Discover - The dynamic nature of the internet may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or name. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided as an educational resources with no clinical information or commercial amalgamation.
- NIH NIH Zebrafish Initiative
- ZFIN - The Zebrafish Model Organism Database
- Keller at European Molecular Biology Laboratory, Germany Movies - Reconstruction of zebrafish early on embryonic development by scanned light sheet microscopy
- YouTube Timelapse recording of virtually 18 hours of embryonic evolution of the zebrafish with some note
Online Atlases
- Fish Face Atlas 3D-interactive atlas of craniofacial development in the zebrafish Danio rerio.
- Zebrafish Atlas
- 3D Atlas of Zebrafish Vasculature Anatomy
- Zebrafish Encephalon Atlas
- Atlas of Zebrafish Anatomy
- Atlas of Zebrafish Evolution
- Zebrafish Anatomy Portal
- FishNet 3D developmental atlas
| Animal Evolution: axolotl | bat | cat | chicken | cow | dog | dolphin | echidna | fly | frog | caprine animal | grasshopper | guinea pig | hamster | horse | kangaroo | koala | lizard | medaka | mouse | opossum | squealer | platypus | rabbit | rat | sea eject | sea urchin | sheep | worm | zebrafish | life cycles | evolution timetable | evolution models | K12 | ||
|
Glossary Links
- Glossary: A | B | C | D | E | F | G | H | I | J | K | L | M | North | O | P | Q | R | S | T | U | V | Westward | 10 | Y | Z | Numbers | Symbols | Term Link
Cite this page: Loma, M.A. (2022, June 22) Embryology Zebrafish Development. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Zebrafish_Development
-
- What Links Here?
- © Dr Marking Hill 2022, UNSW Embryology ISBN: 978 0 7334 2609 4 - UNSW CRICOS Provider Lawmaking No. 00098G
Source: https://embryology.med.unsw.edu.au/embryology/index.php/Zebrafish_Development
Posted by: nicholsdocklinew.blogspot.com


0 Response to "What Do Homeobox Genes Code For In Animals?"
Post a Comment