How Many Pounds Of Nitrogen For Garden
- Home
- Yard and garden
- How-to
- Managing soil and nutrients
- Quick guide to fertilizing plants
Quick facts
- There are 17 essential nutrients that all plants need, including carbon, hydrogen, and oxygen, which plants get from air and water.
- Nitrogen, phosphorus, and potassium are needed in larger amounts than other nutrients; they are considered primary macronutrients.
-
Too much fertilizer is not only a waste of money, but it can damage plants and harm the environment.
-
With the right amount of nutrients, your garden can thrive and provide pounds of produce for harvest.
Plants need nutrients
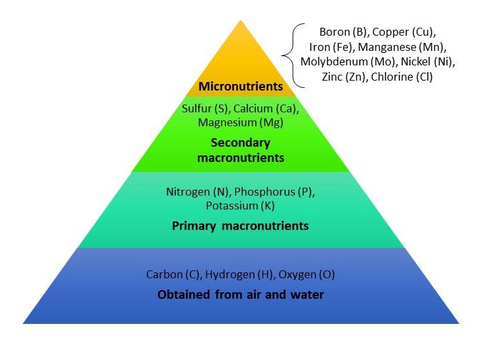
Like us, plants need nutrients in varying amounts for healthy growth. There are 17 essential nutrients that all plants need, including carbon, hydrogen, and oxygen, which plants get from air and water. The remaining 14 are obtained from soil but may need to be supplemented with fertilizers or organic materials such as compost.
- Nitrogen, phosphorus, and potassium are needed in larger amounts than other nutrients; they are considered primary macronutrients.
- Secondary macronutrients include sulfur, calcium, and magnesium.
- Micronutrients such as iron and copper are necessary in much smaller amounts.
Nutrient availability in soils
Nutrient availability in soils is a function of several factors including soil texture (loam, loamy sand, silt loam), organic matter content and pH.
Texture
Clay particles and organic matter in soils are chemically reactive and will hold and slowly release nutrient ions that can be used by plants.
Soils that are finer-textured (more clay) and higher in organic matter (5-10%) have greater nutrient-holding ability than sandy soils with little or no clay or organic matter. Sandy soils in Minnesota are also more prone to nutrient losses through leaching, as water carries nutrients such as nitrogen, potassium or sulfur below the root zone where plants can no longer access them.
pH
Soil pH is the degree of alkalinity or acidity of soils. When pH is too low or too high, chemical reactions can alter the nutrient availability and biological activity in soils. Most fruits and vegetables grow best when soil pH is slightly acidic to neutral, or between 5.5 and 7.0.
There are some exceptions; blueberries, for example, require a low pH (4.2-5.2). Soil pH can be modified using materials like lime (ground limestone) to raise pH or elemental sulfur to lower pH.
Nutrient availability
In general, most Minnesota soils have enough calcium, magnesium, sulfur and micronutrients to support healthy plant growth. Nitrogen, phosphorus, and potassium are the nutrients most likely to be deficient and should be supplemented with fertilizers for optimum plant growth.
The best method for assessing nutrient availability in your garden is to do a soil test. A basic soil test from the University of Minnesota's Soil Testing Laboratory will give a soil texture estimate, organic matter content (used to estimate nitrogen availability), phosphorus, potassium, pH and lime requirement.
The analysis will also come with a basic interpretation of results and provide recommendations for fertilizing.
Choosing fertilizers
There are many options for fertilizers and sometimes the choices may seem overwhelming. The most important thing to remember is that plants take up nutrients in the form of ions, and the source of those ions is not a factor in plant nutrition.
For example, plants get nitrogen via NO3- (nitrate) or NH4+ (ammonium), and those ions can come from either organic or synthetic sources and in various formulations (liquid, granular, pellets or compost).
The fertilizer you choose should be based primarily on soil test results and plant needs, both in terms of nutrients and speed of delivery.
Other factors to consider include soil and environmental health as well as your budget.
Fertilizer analysis
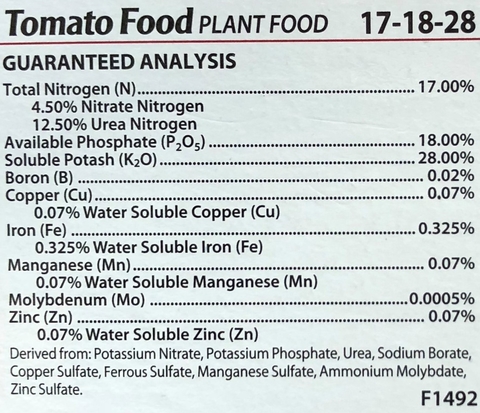
All commercially available fertilizers have what is called a guaranteed analysis, meaning the percent of each major nutrient, by weight, in the fertilizer must be listed on the package as N-P-K.
For example, 10 pounds of 17-18-28 tomato food fertilizer contains 1.7 pounds of nitrogen, 1.8 pounds of phosphorus oxide (P2O5 – phosphate), and 2.8 pounds of potassium oxide (K2O – potash).
Many garden fertilizers contain additional nutrients. Inorganic fertilizers generally list all nutrients on the label, organic fertilizers often contain a wide array of plant nutrients and may not list them all.
If you cannot find a fertilizer with the exact N-P-K ratio that is recommended by your soil test report, you should select a product with a ratio that most closely matches. It's more important to match the nitrogen recommendation than the phosphorus or potassium recommendations, but try not to exceed the phosphorus recommendation because of water quality concerns.
Note that a 10-20-10 fertilizer has a ratio of nutrients that is 1:2:1, meaning that for every 1 pound of N, there are 2 pounds of P2O5 and 1 pound of K2O.
Inorganic vs. organic fertilizers
Fertilizers are either inorganic or organic.
- Inorganic fertilizers do not contain carbon and are generally manufactured, water-soluble products.
- Organic fertilizers are carbon-based and derived from living organisms, such as fish emulsion or blood meal.
- Urea (common in inorganic fertilizers) is a synthetic organic compound; it contains carbon, but is manufactured from inorganic materials and does not qualify for certified organic production.
Inorganic fertilizers
- Can provide rapid delivery of nutrients in liquid form or release a balance of nutrients over a longer period of time in slow-release forms (e.g. slow dissolving or coated).
- Are generally cheaper than organic fertilizers per pound of nutrient.
- Are easy to use when calculating precise application rates.
- Have higher burn potential for plants and greater potential for loss to the environment through leaching or runoff.
- Examples: All-purpose (10-10-10), tomato fertilizer (17-18-28), slow-release granular (15-9-12).
Organic fertilizers
- Are generally considered to be slow-release. Microorganisms in the soil transform organic nutrients into plant-available forms, and this can take days to weeks.
- Are more expensive than inorganic fertilizers per pound of nutrient, but many can be used for certified organic production (read label to be sure).
- May be difficult to calculate precise nutrient content and application rates (as with compost).
- Have lower burn potential and are less likely to be lost to the environment.
- May contain weed seeds or human pathogens like E. coli or Salmonella. Both are found in fresh or improperly composted manure, among other sources.
- Examples: Blood meal (13-2-0), organic tomato fertilizer (3-6-4), fish fertilizer (5-1-1).
Additional benefits of organic materials

Regardless of which fertilizers you choose, consider adding additional organic materials to support overall soil health. Carbon-based materials such as organic fertilizers, compost, grass clippings or cover crops provide a range of benefits to both plants and soil.
- Many organic materials contain other essential plant nutrients in addition to N, P and K.
- Organic materials provide carbon to feed soil microorganisms, increase soil organic matter, and improve overall soil health.
- Adding organic materials to soil also improves water holding capacity in sandy soils and increases drainage and aeration in clay soils.
- Using organic materials as mulch can suppress weed growth, reduce temperature and moisture fluctuations, prevent transmission of soil-borne plant pathogens, and minimize soil erosion.
- Incorporating organic materials can actually reduce the need for supplemental fertilizers.
- Organic materials provide nutrients as they decompose and the resulting soil organic matter will hold and slowly release nutrients over time.
Common nutrient issues in vegetables
Diagnosing nutrient deficiencies or excesses in fruits and vegetables is challenging. Many nutrient issues look alike, often more than one nutrient is involved, and the reasons for them can be highly variable.
Here are some examples of issues you may see in the garden.
- Plants lacking nitrogen will show yellowing on older, lower leaves; too much nitrogen can cause excessive leafy growth and delayed fruiting.
- Plants lacking phosphorus may show stunted growth or a reddish-purple tint in leaf tissue.
- A potassium deficiency can cause browning of leaf tissue along the leaf edges, starting with lower, older leaves.
- A calcium deficiency often leads to "tip burn" on younger leaves or blossom end rot in tomatoes or zucchini. However, calcium deficiencies are often not a result of low calcium in the soil, but are caused by uneven watering, excessive soil moisture, or damage to roots.
- Lack of sulfur on sandy soils can cause stunted, spindly growth and yellowing leaves; potatoes, onions, corn and plants in the cabbage family tend to be most sensitive.
General guidelines for fertilizer use

Nitrogen
- Because nitrogen promotes leafy growth, too much nitrogen late in the season can minimize or delay fruiting.
- Nitrogen is considered to be mobile in the soil, meaning that it moves with water, so it's best to apply nitrogen when plants will be ready to use it.
- Apply nitrogen immediately before or after planting annual crops or following green-up for perennial crops.
- If you have sandy soil, consider using more frequent applications of nitrogen but using a lower application rate.
Phosphorus and potassium
- Among other things, phosphorus supports root growth and fruiting, while potassium supports disease resistance and plant hardiness.
- Phosphorus and potassium are not mobile in the soil and need close contact with plant roots in order to be taken up.
- They should be applied and incorporated prior to planting, either in spring or fall.
Use them wisely
N-P-K fertilizers with ratios of 1:2:2 or 1:2:1 are particularly well-suited for transplants and can be applied when plants are set in the ground.
Foliar fertilizers may also be helpful in some cases, although most nutrient acquisition is through plant roots.
Foliar fertilization may be used to correct deficiencies during the growing season, to provide micronutrients such as zinc or iron when soil pH is greater than 7, or to supply nutrients such as potassium and phosphorus in cool spring soils, when plant roots absorb fewer nutrients
Finally, remember to use fertilizer wisely and always follow label instructions. Too much fertilizer is not only a waste of money, but it can damage plants and harm the environment. With the right amount of nutrients, however, your garden can thrive and provide pounds of produce for harvest.
Rosen, C., Bierman, P., and Finney, H., 2013. Soil Resources and Fertilizers. In Weisenhorn, J. (Ed.), Master Gardener Core Course: Horticulture for the Home Gardener, 41-88. University of Minnesota Extension.
Rosen, C. and Eliason, R., 2005. Nutrient Management for Commercial Fruit & Vegetable Crops in Minnesota. University of Minnesota Extension. http://hdl.handle.net/11299/197955. Accessed 7/2018.
Reviewed in 2020
How Many Pounds Of Nitrogen For Garden
Source: https://extension.umn.edu/manage-soil-nutrients/quick-guide-fertilizing-plants
Posted by: nicholsdocklinew.blogspot.com

0 Response to "How Many Pounds Of Nitrogen For Garden"
Post a Comment